- The FDA has approved a vaccine for RSV that can be given to pregnant women.
- This vaccine can help protect newborns from the highly contagious disease.
- Additionally, a new monoclonal antibody treatment can be given to infants to protect against RSV.
There are now two clear ways to help keep infants protected against RSV: a vaccine for pregnant people and a monoclonal antibody shot approved for all infants.
The Food and Drug Administration (FDA) has approved the RSV vaccine for pregnant people in order to protect their newborn infants against the highly contagious virus.
Previously the vaccine, Abrysvo, was only approved for adults ages 60 and over but is now approved for use at 32 through 36 weeks gestational age of the pregnancy.
This news comes on the heels of the American Association of Pediatrics (AAP) advising that all infants should receive the RSV prevention monoclonal antibody shot, Beyfortus, to help protect them against RSV.
We talked to experts about what parents should know about the virus and how they can protect their kids.
What is RSV?
Respiratory syncytial virus, more commonly known as RSV, is a respiratory infection that causes cold-like symptoms in healthy adults but can be particularly dangerous for young infants and older adults, leading to lower respiratory tract disease that can sometimes be severe.
“Children under age six months, premature infants that require intensive care in the first few weeks or months of life, and children with underlying heart or lung conditions are at extremely high risk to suffer from severe disease,” said Dr. Sara Siddiqui, a pediatrician with NYU Langone Huntington Medical Group.
“RSV is known to cause respiratory symptoms and difficulty breathing in young infants leading to hospitalization and possibly death.”
The new vaccine is given to pregnant people later in their pregnancy in the hopes that newborns will be protected in the first few months of their life.
When a pregnant person gets vaccinated they can pass on key antibodies to children after they are born. For the first few months of a child’s life, these antibodies from their parent can help protect them against disease.
“RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization,” said Dr. Peter Marks, PhD, director of the FDA’s Center for Biologics Evaluation and Research in a statement.
“This approval provides an option for healthcare providers and pregnant individuals to protect infants from this potentially life-threatening disease.”
Preventing RSV in infants
While there are currently no medications to treat RSV, there are ways to help prevent it. Both the Abrysvo vaccine for pregnant people and the Beyfortus monoclonal antibody shot for infants are ways to help keep everyone healthy and safe.
What is the difference between the RSV monoclonal antibody shot and the vaccine?
Monoclonal antibodies are laboratory-made proteins that mimic the immune system’s ability to fight off viruses almost immediately.
Vaccines are meant for long-term protection and prevention.
“Nirsevimab [Beyfortus] is a monoclonal antibody, not a vaccine, given in a single intramuscular shot, which studies have shown provides protection which lasts for 150 days or about five months,” said Dr. Rebecca Fisk, a pediatrician at Northwell Lenox Hill Hospital.
A clinical study evaluated the effectiveness of Abrysvo, the vaccine, to lower respiratory tract disease and severe lower respiratory tract disease caused by RSV in infants born to individuals who were vaccinated during pregnancy.
The study found that Abrysvo reduced the risk of severe lower respiratory tract disease by 82% within 90 days after birth and 69% within 180 days after birth.
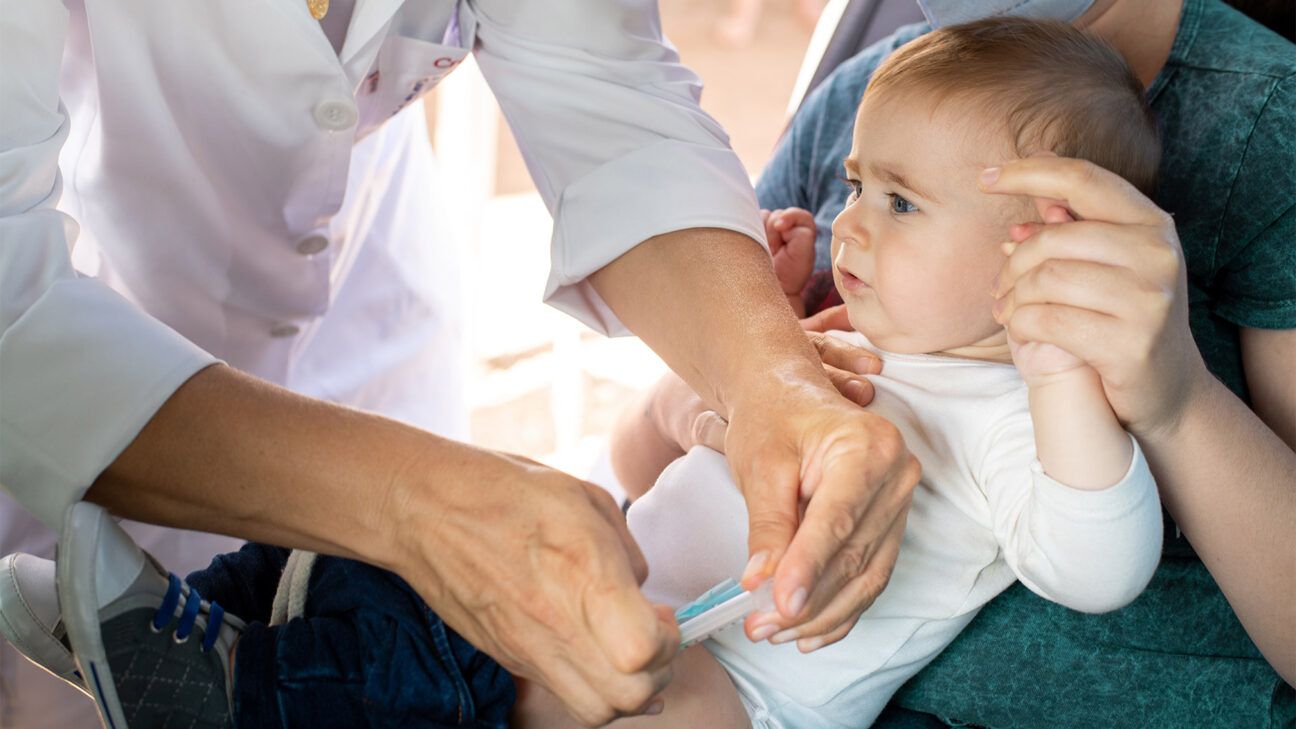
What is the monoclonal antibody treatment?
According to the American Academy of Pediatrics (AAP), a single dose of Beyfortus is recommended for:
- All infants younger than eight months during or entering their first RSV season.
- Infants and children ages eight to 19 months who are at increased risk of severe RSV disease and entering their second RSV season.
The AAP outlines that Beyfortus is an intramuscular injection with a pre-filled syringe. It is given as a single dose to infants at 50 milligrams for children weighing less than 11 pounds and 100 milligrams for children weighing 11 pounds and above. Side effects include rash and injection site reactions.
Beyfortus will be covered by insurance and through the Vaccines for Children (VFC) program, which covers immunizations for children who are Medicaid-eligible, uninsured, or underinsured.
The AAP says the shot is expected to cost $495 in the private sector and $395 in the VFC program. It is unclear if the shot will be included in bundled payments for newborn care.
“There does not seem to be clear information as of yet regarding how nirsevimab [Beyfortus] will be distributed or how private insurers will handle it,” said Fisk.
“VC inclusion means that those children who are eligible will get this through federal funds. Still, there are many unanswered questions regarding how parents will obtain this monoclonal antibody for their infants at a time when RSV season is just around the corner. More information is needed from private and public insurers regarding cost and insurance coverage.”
Should your child get the monoclonal antibody shot for RSV?
The Beyfortus shot is an effective method for helping to keep infants safe. Efficacy was about 81% against hospitalization, according to the Centers for Disease Control and Prevention (CDC).
“I will be discussing it with my patients and families regarding their risks of all respiratory illnesses in the coming months,” said Siddiqui.
“I will include conversations about specific risks and benefits for patients and how best to protect their infant from RSV and other viruses. The RSV protection will be one of the tools we can now recommend to help to reduce hospitalizations in our children.”
Takeaway
The Food and Drug Administration (FDA) has approved the RSV (respiratory syncytial virus) vaccine for pregnant people to protect their infants against the highly contagious virus.
In addition, a monoclonal antibody shot can now be given to infants to protect them against the virus.
FDA Approves Vaccine for RSV to Protect Newborns: What to Know
Source: Pinoy Lang Sakalam



0 (mga) komento:
Mag-post ng isang Komento